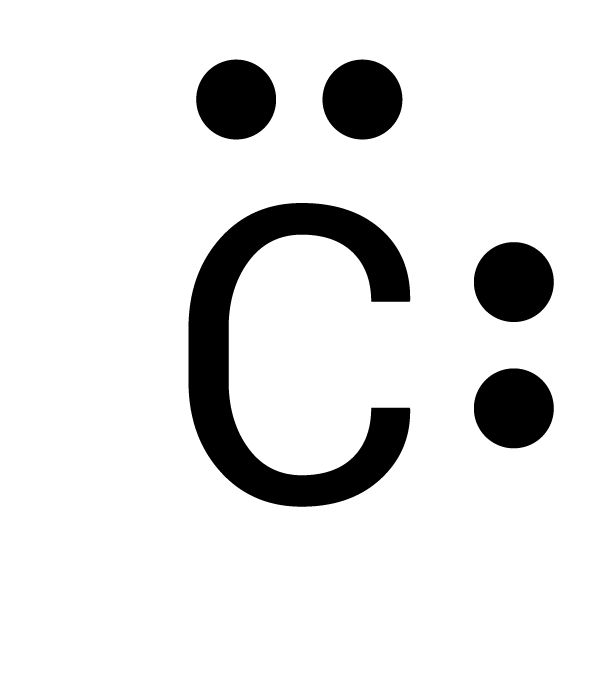Are you ready to take this lesson's quiz? These questions will help you find out. Make sure you understand why each correct answer is correct--if you don't, review that part of the lesson.
What are the possible values of the magnetic quantum number (mℓ) for an electron with an angular quantum number of 2 (ℓ=2)?
- 0, 1
- 0, 1, 2
- -1, 0, 1
- -2, -1, 0, 1, 2
The magnetic quantum number has the possible values equal to - ℓ to + ℓ.
The value ℓ for an electron is n-1, while the values for mℓ are equal to - ℓ to + ℓ.
The possible values for mℓ are equal to - ℓ to + ℓ. Since ℓ=2, the range of values are integers from -2 to 2.
The possible value for mℓ is equal to - ℓ to + ℓ, which is -2, -1, 0, 1, 2.
What statement supports Hund's rule for electron filling?
- Electrons will always fill the lowest energy orbitals first.
- All energy orbitals with the same energy must get one electron before receiving a second.
- Electrons in the same electron orbital may have a negative orientation (-½) and a positive orientation (+½).
- Fill the lowest energy orbital first of the orbital-filling chart before the next energy level.
Aufbau's principle states that.
Hund's rule states that every orbital of the same energy must have one electron before any orbital gets a second.
The Pauli exclusion principle states that no two electrons in the same atom can have identical numbers.
The orbital-filling chart applies Aufbau's principle to guide electron filling.
Which represents the correct order of energy levels?
- 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
- 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6
- 1s2 2s2 2p6 3s2 3p6 4s2 4p6 3d10
- 1s2 2s2 2p6 3s2 3p6 3d10 4p6 4s2
4s has a lower energy level than 3d. And 3d has a lower energy level than 4p.
4s has a lower energy level than 3d.
3d has a lower energy level than 4p but higher than 4s.
4s has a lower energy level than 3d.
What is the shorthand electron configuration for phosphorus?
- [Ar] 3s2 3p3
- [Ne] 3s2 3p3
- [Ar] 3s2 3p6
- [Ne] 3s2 3p6
Argon is the noble gas after--not before--phosphorus.
The noble gas before phosphorus is neon. And the configuration of the valence electrons is 3s2 3p3.
Argon is the noble gas after--not before--phosphorus.
The noble gas before phosphorus is neon. But phosphorus has five valence electrons instead of eight.
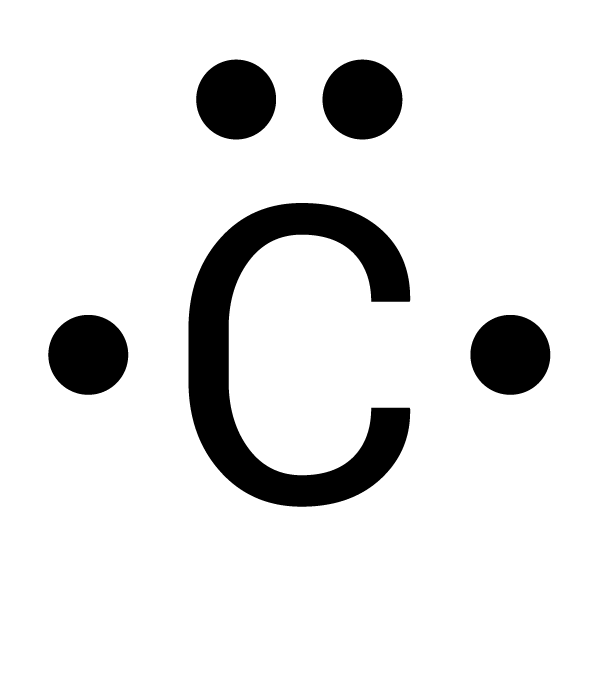
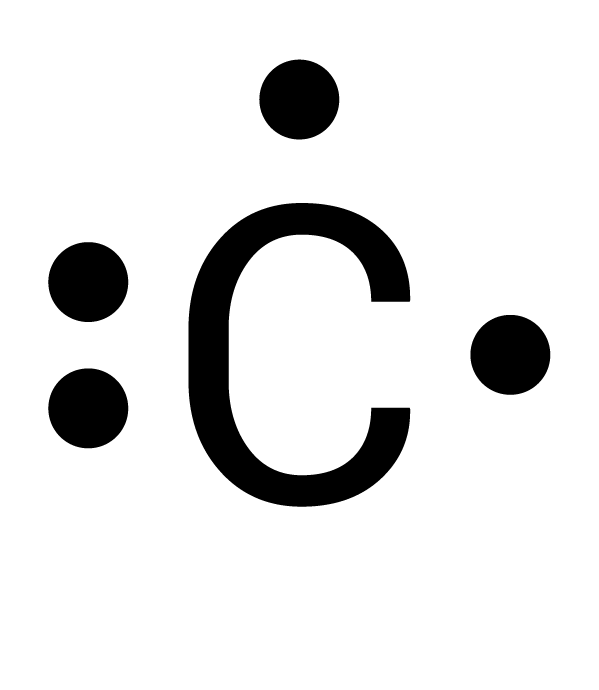
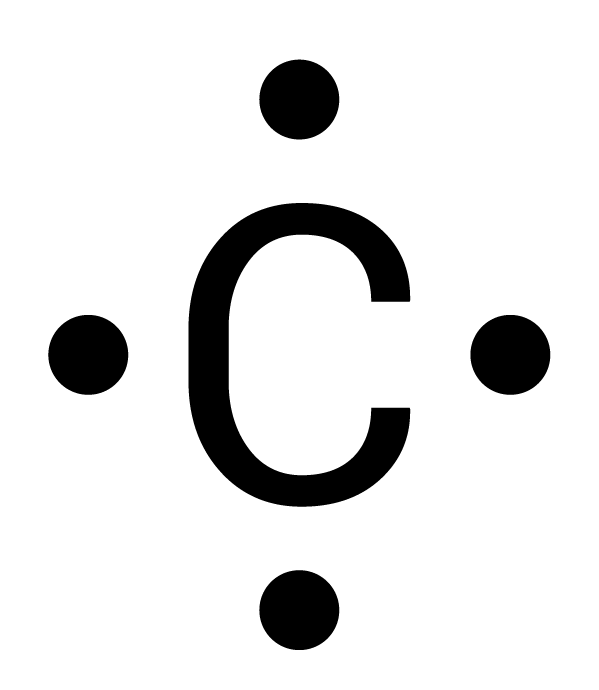
Which is the correct electron-dot structure for carbon (C)?
The electrons must be spaced out to each of the four sides before doubling up.
Two electrons are spread out. Each of the four electrons must be spaced out to each of the four sides before doubling up.
Two electrons are spread out. Each of the four electrons must be spaced out to each of the four sides before doubling up.
All of the four electrons are separated based on Hund's rule.
Summary
Questions answered correctly:
Questions answered incorrectly: