Electrons follow certain rules when filling atomic orbitals. Complete the activities below to see how well you understand the rules used to describe how electrons fill atomic orbitals.
In the first activity, use the Aufbau principle to place the energy levels in the correct order from top to bottom, beginning with 1s2 at the top. Keep trying until the orbitals appear in the correct order.

1s2
2s2
2p6
3s2
3p6
4s2
3d10
4p6
5s2
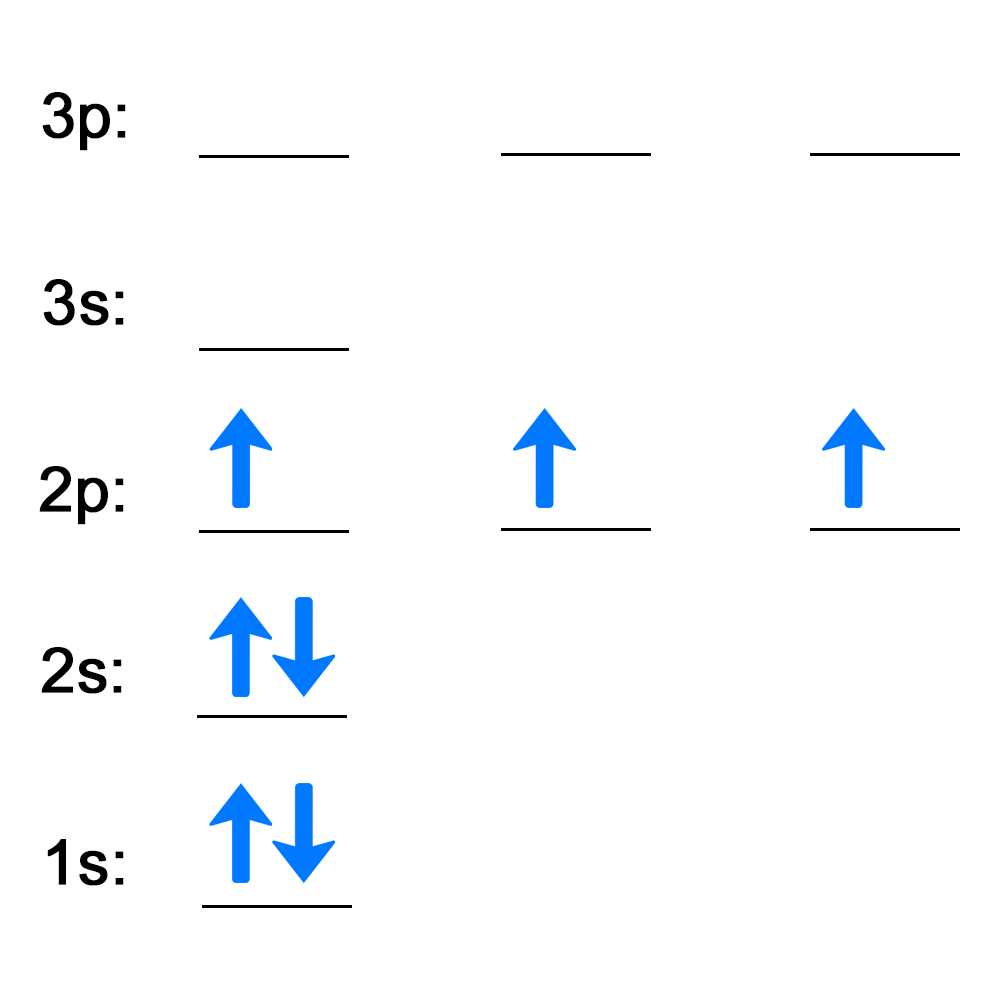
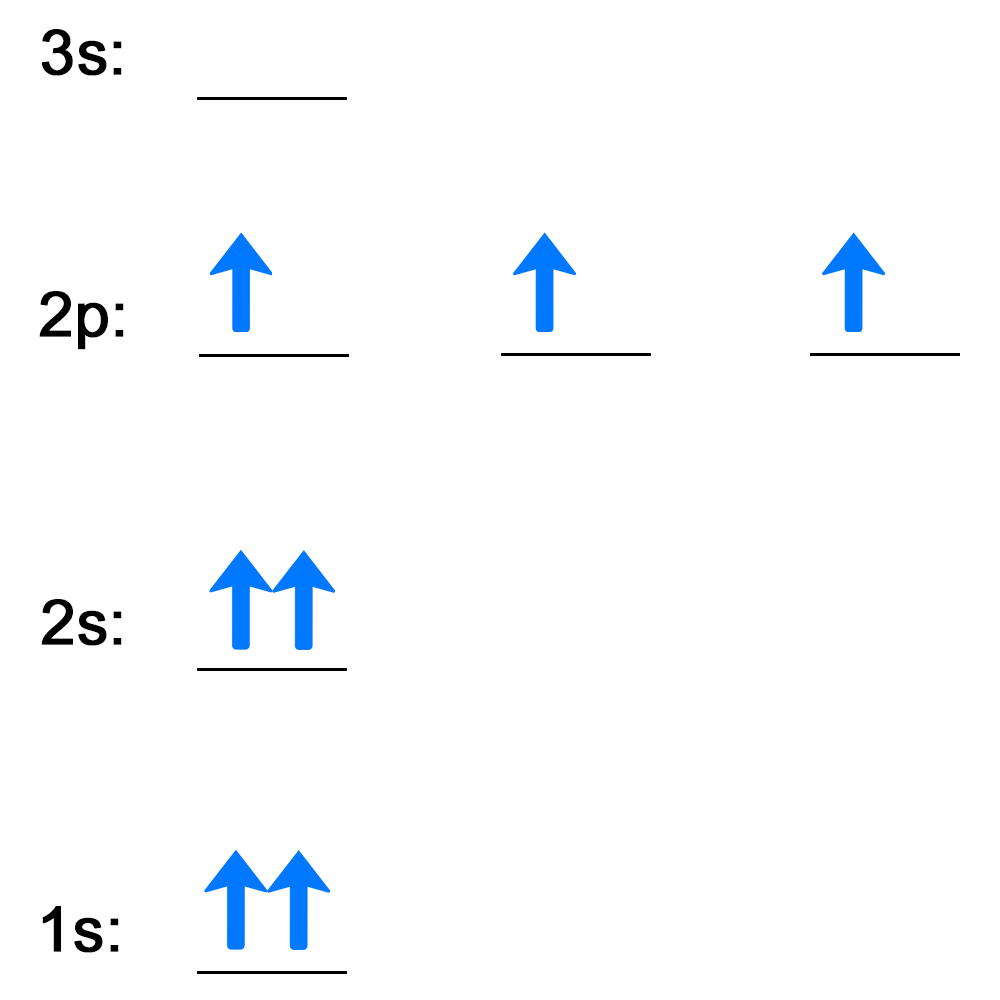
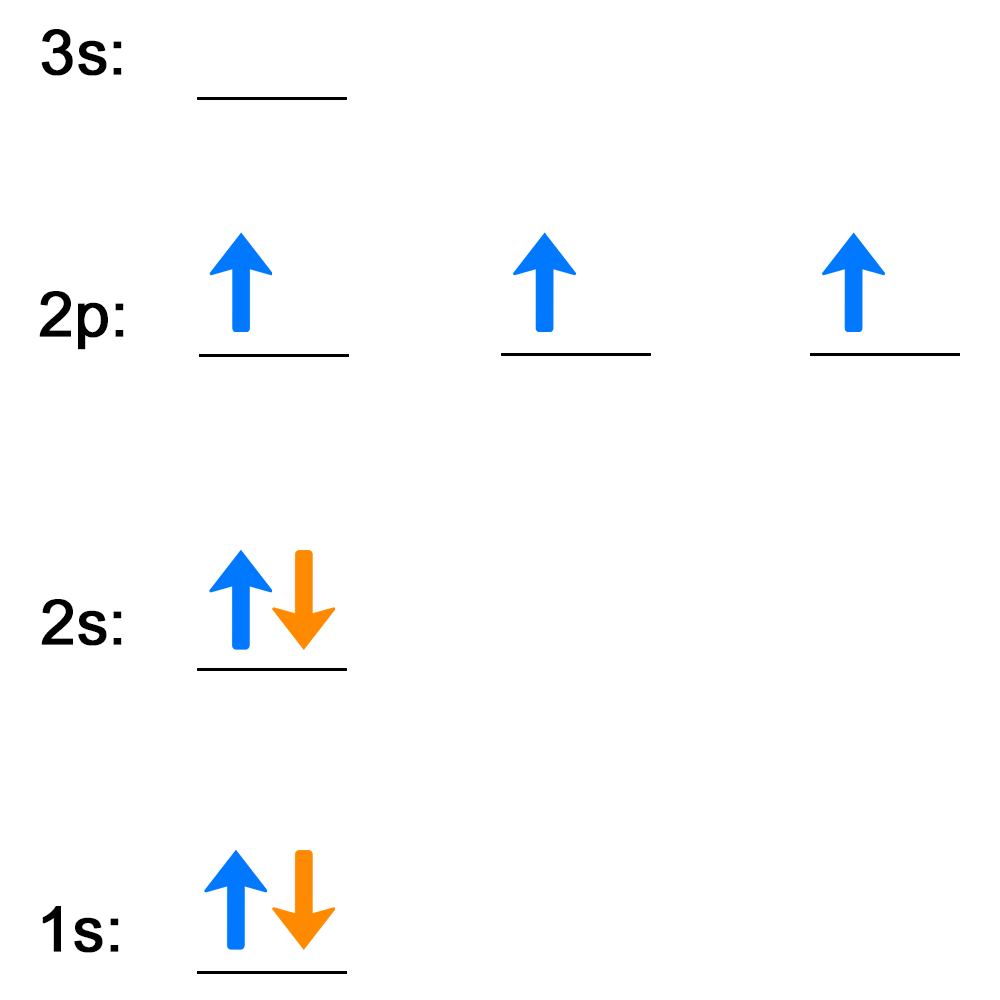
Now, see how well you understand Hund's rule and the Pauli exclusion principle by completing this activity. Answer the question on each slide, then check your answer.