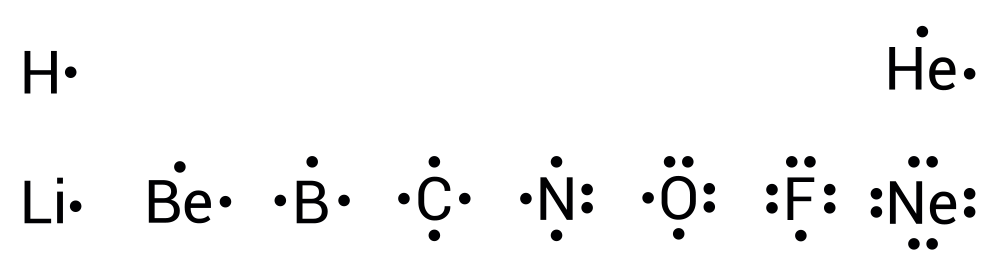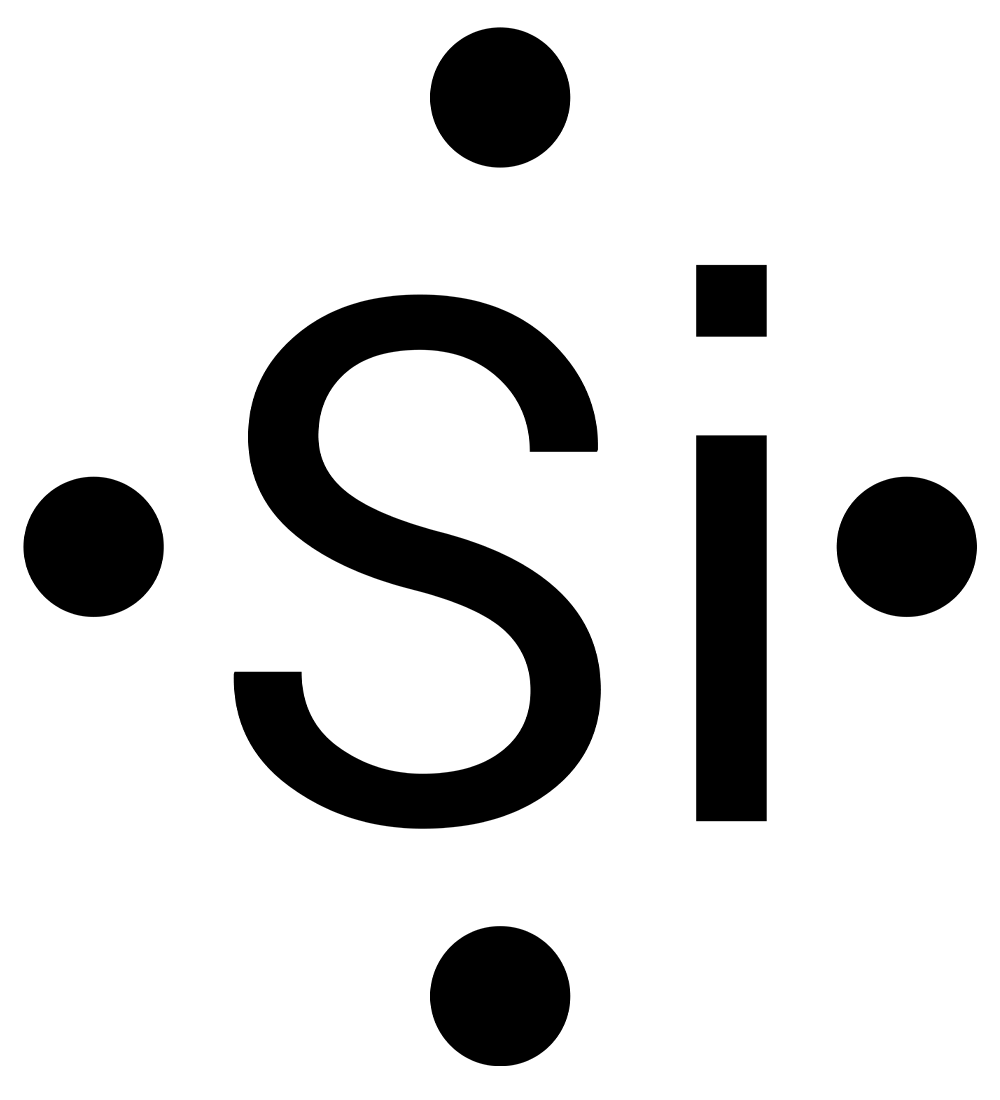The chemical properties of an element depend on the number of valence electrons, and the valence electrons are the electrons that are involved in forming chemical bonds. Therefore, it is useful to have a simple shorthand method to represent them visually. This is done using an electron-dot structure. An electron-dot structure (also called an electron-dot diagram) is a diagram in which each dot represents a valence electron.
An electron-dot structure consists of the element's symbol (which represents the atomic nucleus and inner-level electrons) surrounded by dots that represent the atom's valence electrons. Just as a Bohr model can be used to represent all of the electrons in an atom, an electron-dot structure can be used to focus on only the valence electrons. Look at this example.
The Bohr model of oxygen shows all of the electrons in the atom, including its valence electrons. Drawing an electron-dot structure represents only the valence electrons.

The steps for drawing an electron-dot structure that represents the number of valence electrons in an atom are shown in the table below. Click each step to see it applied to this example.
|
The symbol in the center represents the nucleus and all the other electrons in the atom. 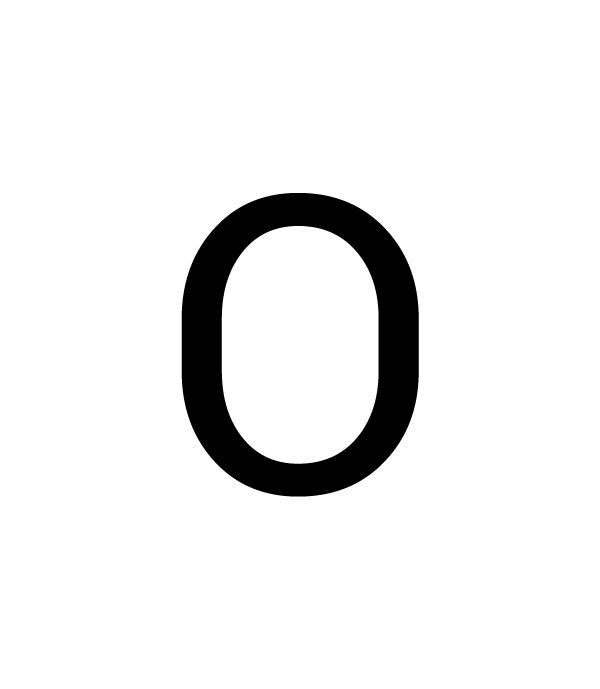
|
|
|
There are different ways to determine the number of valence electrons. One way is by writing its electron configuration. The atomic number of oxygen is 8, so it has eight electrons. Its electron configuration is 1s2 2s2 2p4. For oxygen, the outermost energy level is 2. There are two electrons in 2s and four electrons in 2p for a total of six valence electrons (2 + 4 = 6). Another way is to use the periodic table of elements. Oxygen is in Group 16. For the elements in Groups 13-18, subtract 10 from the Group number to find the number of valence electrons. For example, oxygen is in Group 16 and has six valence electrons (16 - 10 = 6). |
|
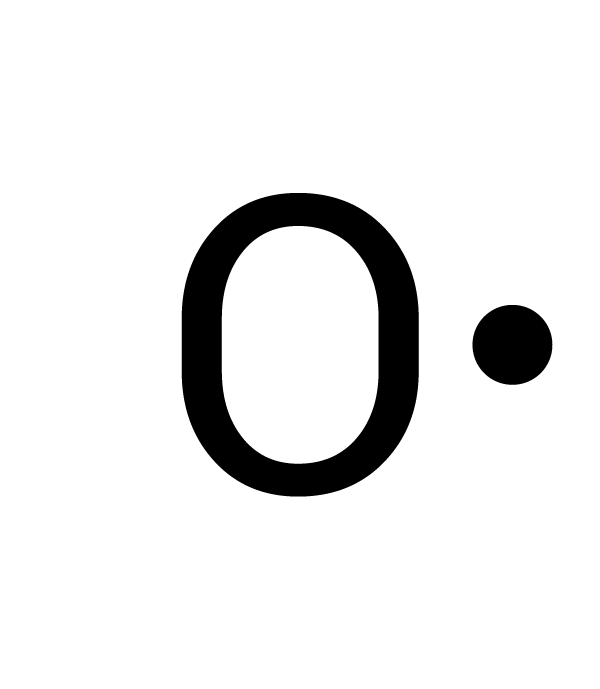
|
|
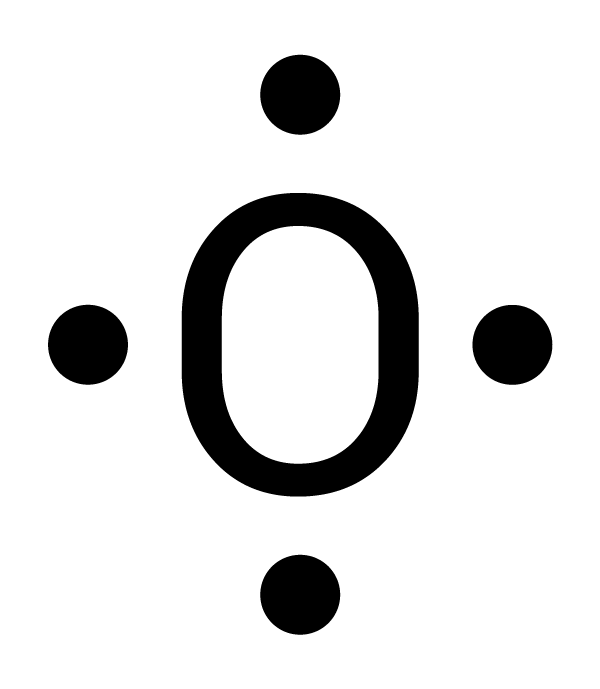
|
|
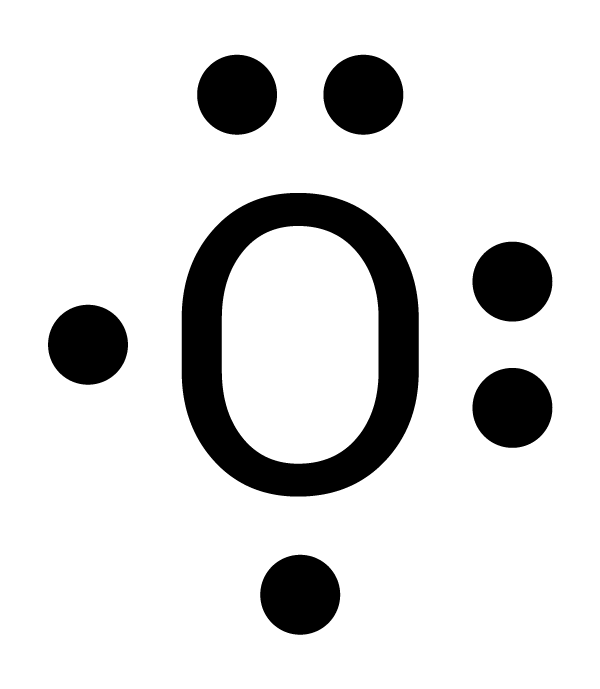
|
Here are the electron-dot structures for the first 10 elements on the periodic table. Notice that, with the exception of helium, elements in the same group have the same number of dots in their electron-dot structures because they have the same number of valence electrons.
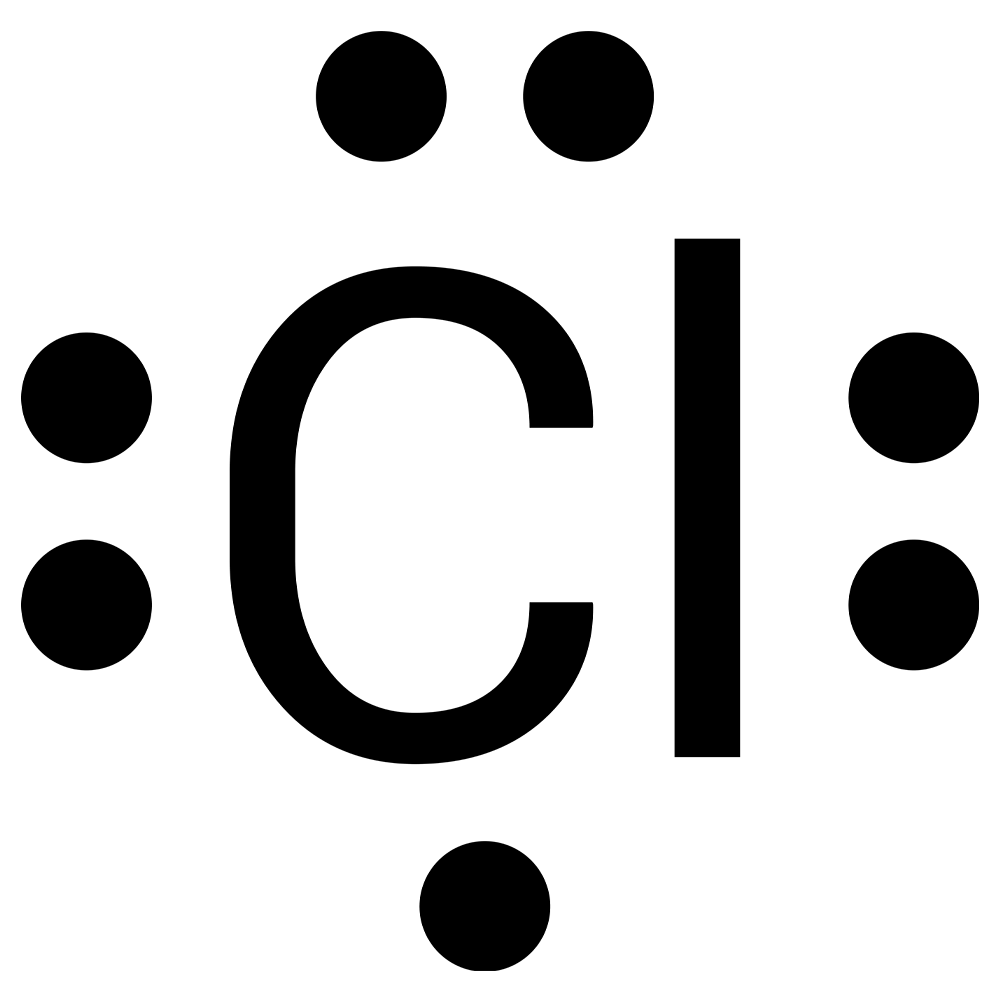
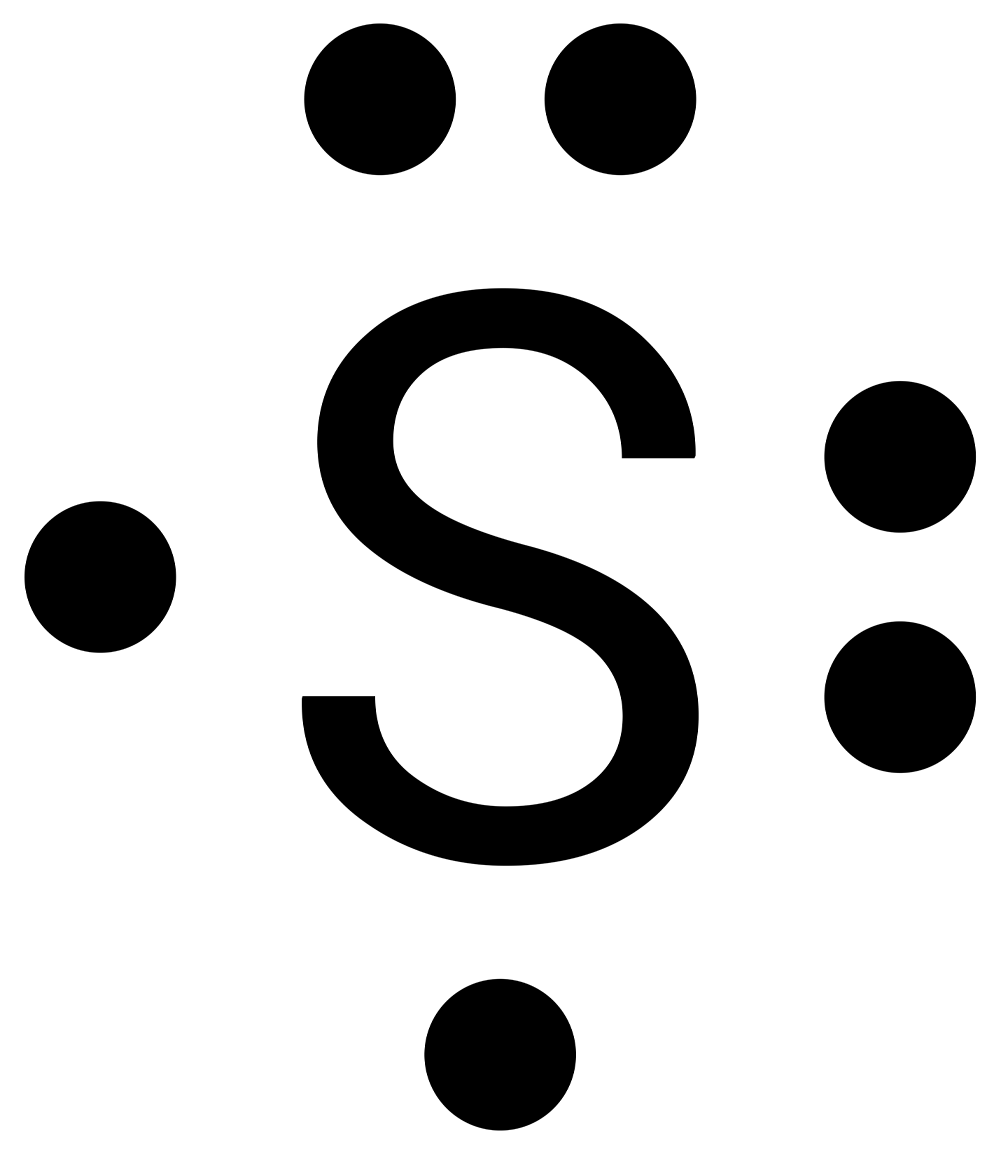
Complete this activity to practice drawing electron-dot structures. Draw the electron-dot diagram for the element on each slide, then check your answer.
If you need a periodic table, click below to open an interactive periodic table or to download a PDF.