In a previous lesson, you learned that--according to the periodic law--when atomic numbers are used to arrange elements into groups, properties repeat in a predictable way. From this lesson, you have learned that elements in a group have similar electron configurations. Since an element's electron configuration determines its chemical properties, elements in a group have similar chemical properties.
The chemical properties of an element are determined by the valence electrons. The valence electrons are the electrons that are in an atom's outermost or highest occupied energy level. Study the examples on each tab to learn how to determine the number of valence electrons an element has.
You can determine the number of valence electrons an element has by writing its electron configuration. For example:
The Bohr model of sulfur shows that sulfur has six valence electrons. These are easy to see highlighted in the image. How can we determine the number of valence electrons sulfur has by writing its electron configuration?
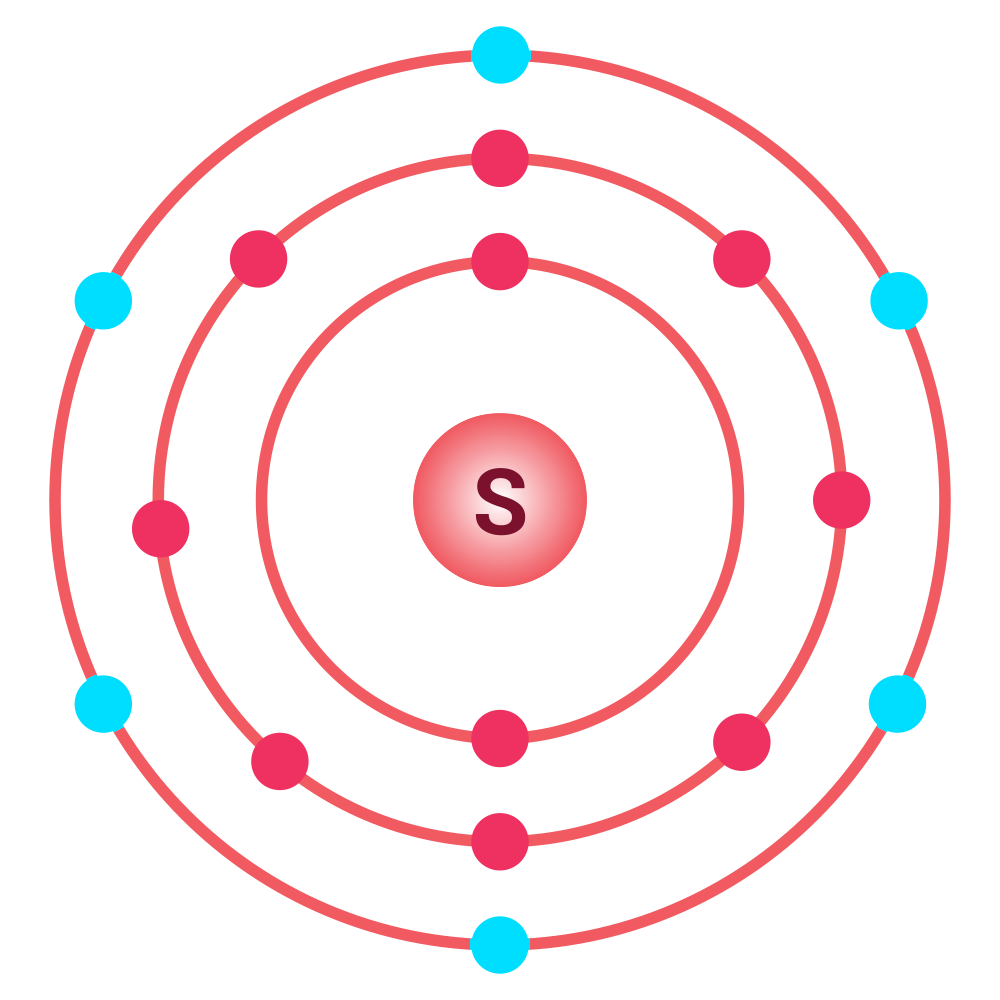
The electron configuration for sulfur is 1s2 2s2 2p6 3s2 3p4. The last principal energy level (n) is also the valence energy level because it is the outermost energy level.
1s22s22p63s23p4
For sulfur, the outermost energy level is 3. There are two electrons in 3s and 4 electrons in 3p for a total of six valence electrons (2 + 4 = 6).
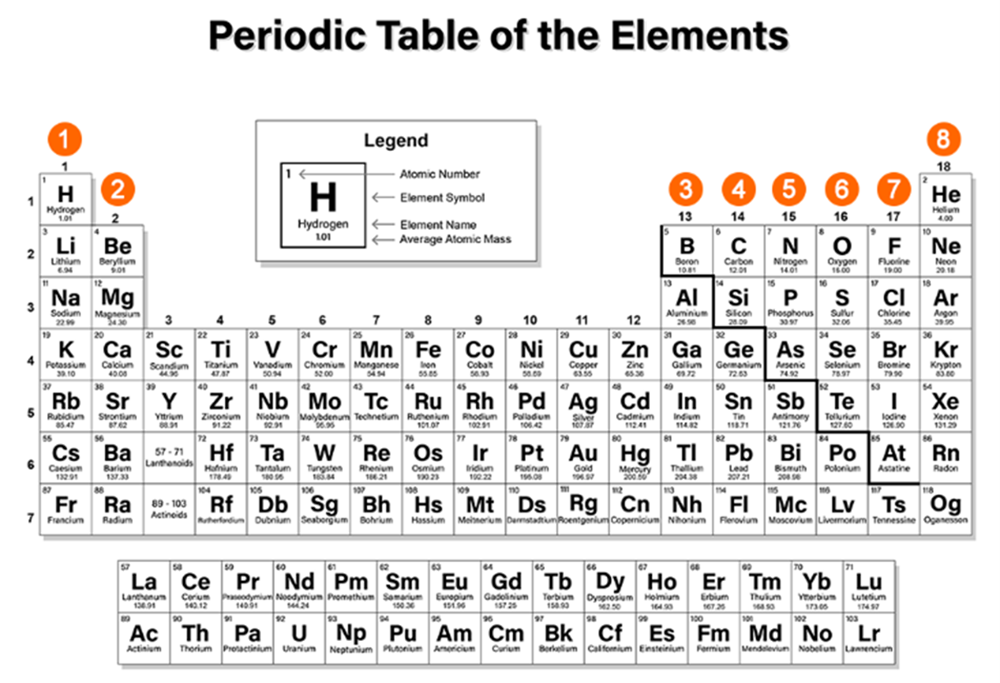
Another way to determine the number of valence electrons an element has is by noting its position on the periodic table.
There are 18 groups on the periodic table. Recall that the elements in Groups 1-2 and 13-18 are called the representative elements, or main group elements. A representative element's group number and the number of valence electrons its atoms contain are related.
This periodic table shows how a representative element's group number and the number of valence electrons its atoms contain are related. Click the periodic table to learn about this relationship.
Calcium sulfide (CaS) is a solid compound that is used in the production of certain types of paints, ceramics, and paper. Locate calcium and sulfur on the periodic table. How many valence electrons do calcium and sulfur have?

Calcium (Ca) is in Group 2 and has two valence electrons.
Sulfur (S) is in Group 16 and has six valence electrons. For the elements in Groups 13-18, subtract 10 from the Group number to find the number of valence electrons. For example, oxygen is in Group 16 and has six valence electrons (16 - 10 = 6).
Complete this activity to see how well you can determine the number of valence electrons a representative element contains. Use the periodic table to match each element on the left with the correct number of valence electrons on the right.
If you need a periodic table, click below to open an interactive periodic table or to download a PDF.