The assignment for this project is to plan and conduct a lab experiment that infers information about the structure of a substance and the electrical forces between the particles of the substance.
One simple experiment that you can do to learn about the forces between particles in common substances is shown in this image. First, water is put on a plate or in a bowl. Next, pepper is sprinkled on top of the water. Finally, a drop of detergent is dropped in the middle of the water.
The result is shown in the image. All the pepper quickly moves to the outer edge of the plate. However, if you try the experiment again with the same water but just add more pepper, nothing happens.
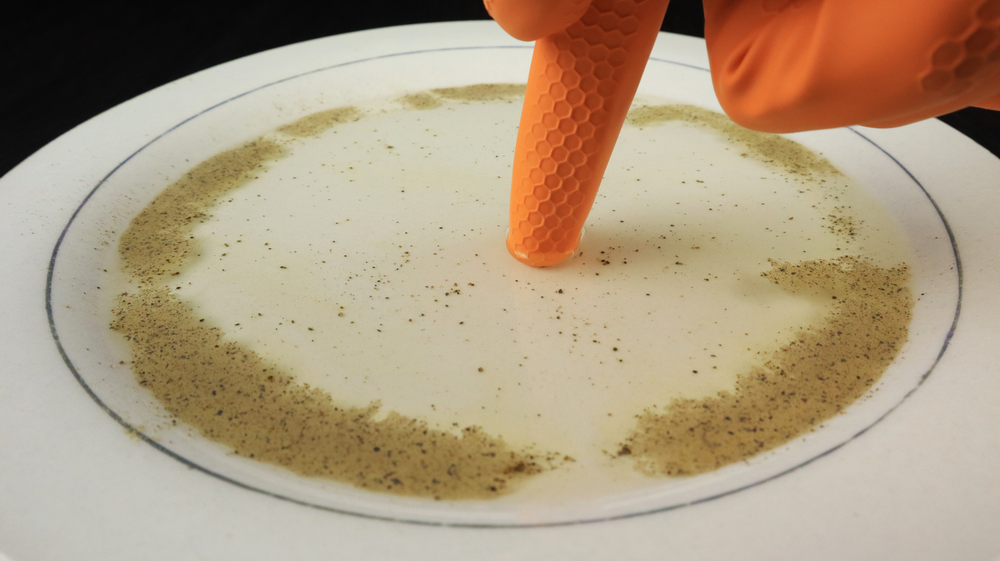
Why does pepper behave the way it does? Well, that has to do with polar and non-polar molecules, which you will review in this lesson.
Some other kitchen substances to test could be salt and sugar. Or you could change the detergent with other substances like oil or milk. Do you think other substances will behave in this same way? You will find out when you conduct your investigation!
Question
What do you remember about polar and nonpolar molecules?
Polar molecules have ends with slightly different charges, and nonpolar molecules do not have ends with different charges.
Why do you think pepper behaved the way it did in this experiment? What do you think sugar and salt would do?


