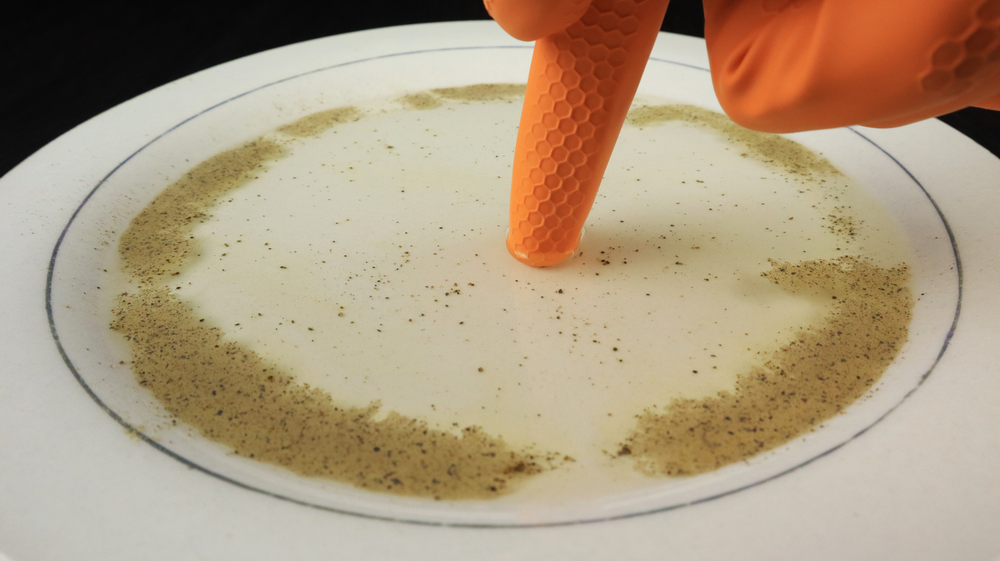
Notice that in the pepper and water experiment, the pepper is still visible; it has not dissolved in the water. This can be explained by polarity.
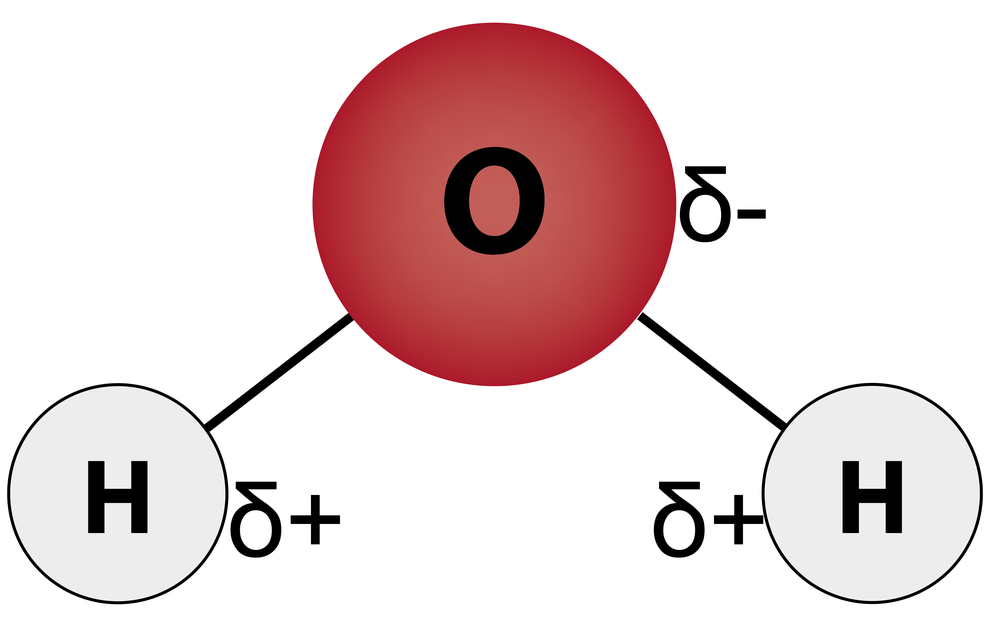
In a previous lesson, you learned that water is a polar molecule, so it has a partial positive and a partial negative charge on opposite sides of the molecule.
Why is water polar?
Recall that electronegativity refers to how strongly an atom pulls electrons towards itself in a chemical bond. A polar covalent bond is formed between two atoms of similar but not equal electronegativities. Because they have a small difference in electronegativity, neither atom is “strong” enough to attract the electrons away from the other atom. Therefore, they end up sharing their valence electrons, but it is an unequal sharing.
Water has bonds between the oxygen atom and the two hydrogen atoms. Oxygen is more electronegative than hydrogen, so oxygen pulls on the electrons more towards itself. This gives the oxygen a partial negative charge and the hydrogens a partial positive charge.
Bonds that show this separation of charges are called dipoles. These slight charges are represented by the Greek letter delta (δ). Only polar covalent bonds form dipoles. Because these bond dipoles do not cancel each other out, the overall molecule is polar, with a partial negative charge on oxygen and a partial positive charge on each hydrogen.
You have also learned that the common phrase when discussing substances dissolving is “like dissolves like.” This means that generally, polar substances will dissolve in other polar substances and nonpolar substances will mix with nonpolar substances. Recall that water is often called the universal solvent because many things will dissolve in it. This occurs because water is a polar molecule and substances that have charges will dissolve in it.
Read over this table to review what you have already learned about the dissolving of different substances in water.
| Ionic Substances | Ionic compounds form from positive and negative ions. When ionic compounds are put in water, they dissociate (or break up) into the positive and negative ions. These will be attracted to the positive and negative charges on the water. |
| Polar Substances | Polar compounds do not dissociate into ions, but they will dissolve in water because they have positive and negative ends like the water does. The positive ends are attracted to the partially negative ends of water and the negative ends are attracted to the partially positive ends of water. |
| Nonpolar Substances | Nonpolar molecules do not have bonds with different electronegativity between atoms. As a result, they do not form positive and negative ends, and their molecules are not attracted to each other as much as polar ones are. Because they do not have ions or partial charges, they will not interact with water |
Hydrophobic and Hydrophilic Substances
Substances that can dissolve in water are called hydrophilic. These materials have a special affinity for water. Some common kitchen substances that are hydrophilic are sugar which is polar covalent and salt which is ionic.
Substances that are nonpolar will not dissolve or mix with water. These are called hydrophobic. Some common substances that are hydrophobic are grease, wax, oil, and other fats.
Question
Based on what you have learned about hydrophobic and hydrophilic substances, why would pepper not dissolve in the water?
Since pepper does not dissolve in water, it is hydrophobic.
The question about why pepper does not dissolve in water was answered, but why does it float on the surface of water?

