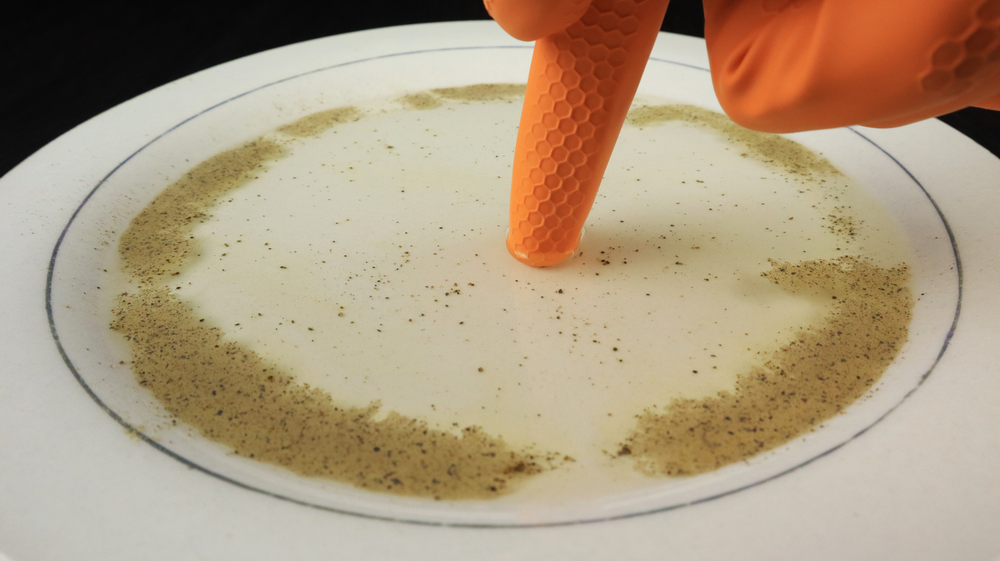
Notice that in the pepper and water experiment, the pepper floats on the surface of the water. It floats because of the surface tension of the water. Recall that surface tension is the resistance of a liquid to spread out and increase its surface area.
Why does water have a high surface tension?
You have learned that each water molecule has a partial negative charge on oxygen and a partial positive charge on each hydrogen. In a sample of water, the partially positive hydrogen on one water molecule will be attracted to the partially negative oxygen of another water molecule. This creates intermolecular forces of attraction called hydrogen bonds.
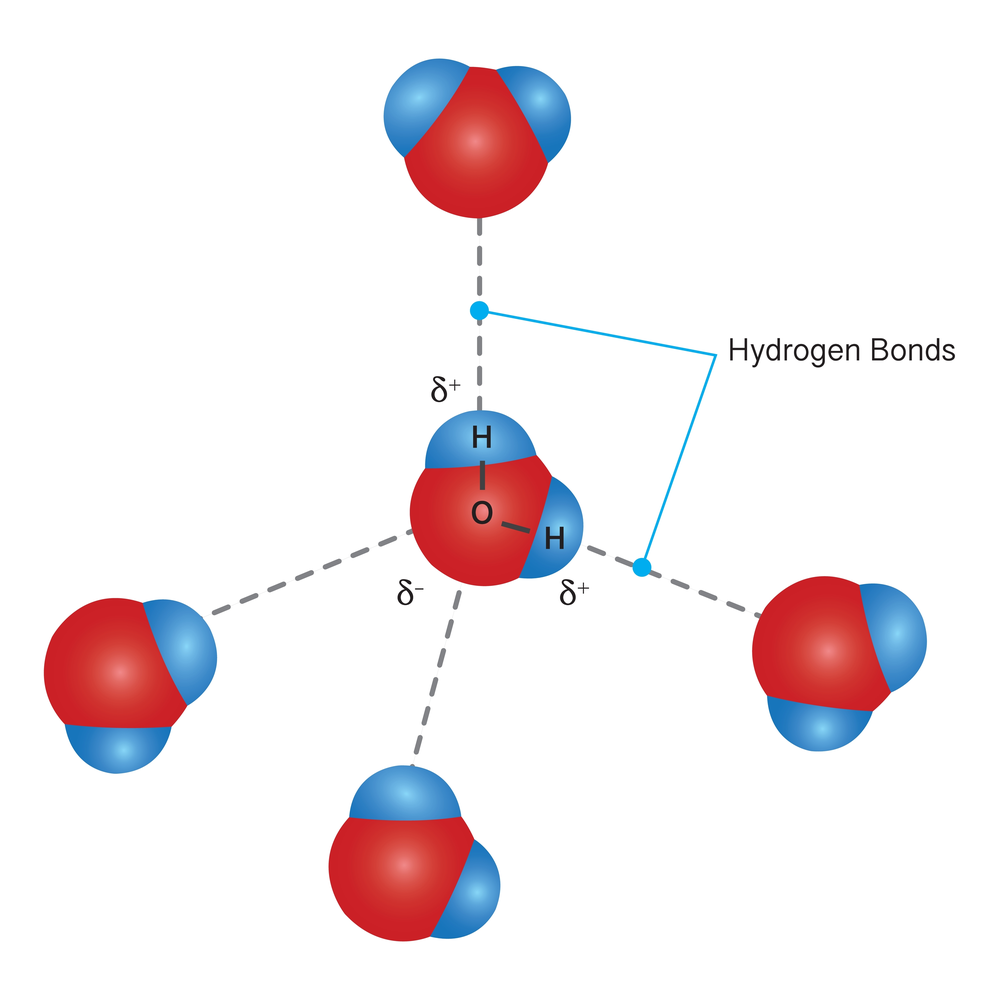
These attractions hold the water molecules together. The molecules at the surface do not have as many water molecules to cling to since there are none above them. Because of this, there is a stronger attraction between those molecules that are in contact at the surface of the water. This forms a surface that resists external forces and thus water has a higher surface tension than any other liquid.

1 row of blue balls and 3 rows of light blue balls. Arrows pointing from dark blue balls inward to other balls. Some light blue balls have arrows pointing out in every direction. Text says Molecules on the surface are attracted to one side. Molecules in the middle are attracted equally in all directions.
Now we have enough information to explain the experiment with the pepper and the water. Since pepper is hydrophobic, it does not dissolve in water. It is also light enough that it does not exert enough force to break through the surface tension of the water, so it floats on the surface of the water.
What about the detergent?
What is the reason for the pepper's behavior after the detergent is added to the water? Detergents decrease the surface tension of water by disrupting the hydrogen bonds between the water molecules. When the hydrogen bonds are broken, the water spreads out.
In the pepper and water experiment, when the surface tension of the water is broken, the water molecules move away from the detergent and carry the pepper with them.
Question
Would nonpolar substances have the same type of surface tension as water?
No. Nonpolar substances do not have the attractions between molecules that water does, so they do not have surface tension like water does.
How could the pepper and water experiment be changed to test other substances that would break the surface tension of water?

