Complete this activity to practice classifying organic compounds. Identify what class the organic compound on each slide belongs to, then check your answer.

Alcohol
There is a hydroxyl functional group (-OH) at the end of the carbon chain.

A molecule consisting of a chain of carbon atoms. A carbon atom toward the end of the chain is double bonded to an oxygen atom and single bonded to an oxygen that is then single bonded to another carbon.
Ester
This molecule has an ester functional group, which is both a single bonded and double bonded oxygen within the carbon chain.

Alkene
This molecule has a double bond located between the third and fourth carbon atoms.

A molecule consisting of a chain of carbon atoms. A carbon atom at the end of the chain is double bonded to an oxygen atom and single bonded to a hydroxyl (OH-) group.
Carboxylic Acid
There is both a carbonyl (=O) and hydroxyl (-OH) group at the end of the carbon chain.
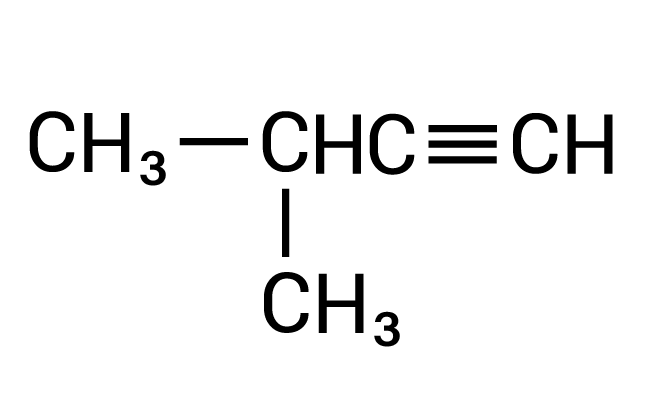
Condensed structural formula of 3-methyl-1-butyne showing a 4-carbon hydrocarbon chain with a triple bond between the first and second carbon atom. There is a 1-carbon branch at the third carbon atom in the chain.
Alkyne
This molecule has a triple bond located between the first and second carbon atoms.

A molecule consisting of three carbon atoms. The carbon atom at the end of the chain is double bonded to an oxygen atom and single bonded to a hydrogen atom.
Aldehyde
This molecule has a carbonyl group (=O) located at the end of the carbon chain.

Ketone
This molecule has a carbonyl group (C=O) located in the middle of a carbon chain.
Slide:
