We know that all matter is made up of particles. The particles that make up matter are so small, that even the smallest sample contains an extremely large number of them.
Because of the difficulty in counting these very small particles, chemists devised a convenient counting unit called the mole. The mole, abbreviated as mol, is the SI base unit used to measure the amount of a substance. The quantity one mole is set by defining one mole of carbon-12 atoms to have a mass of exactly 12 grams.
A mole of anything contains 6.02214076 × 1023 representative particles. A representative particle is any kind of particle such as atoms, molecules, or formula units. Some examples of representative particles are shown in this table.
| Representative Particle | Example |
|---|---|
Atom |
The representative particle in a mole of sodium (Na) is the sodium atom.  |
Molecule |
The representative particle in a mole of water (H2O) is the water molecule. 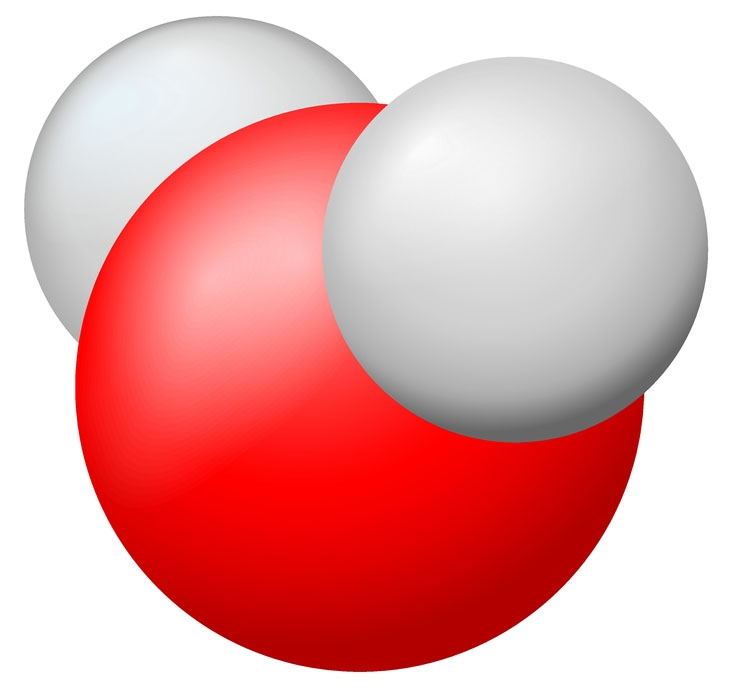 |
Formula Unit |
The representative particle in a mole of sodium chloride (NaCl) is the formula unit, which consists of one Na+ ion and one Cl− ion. 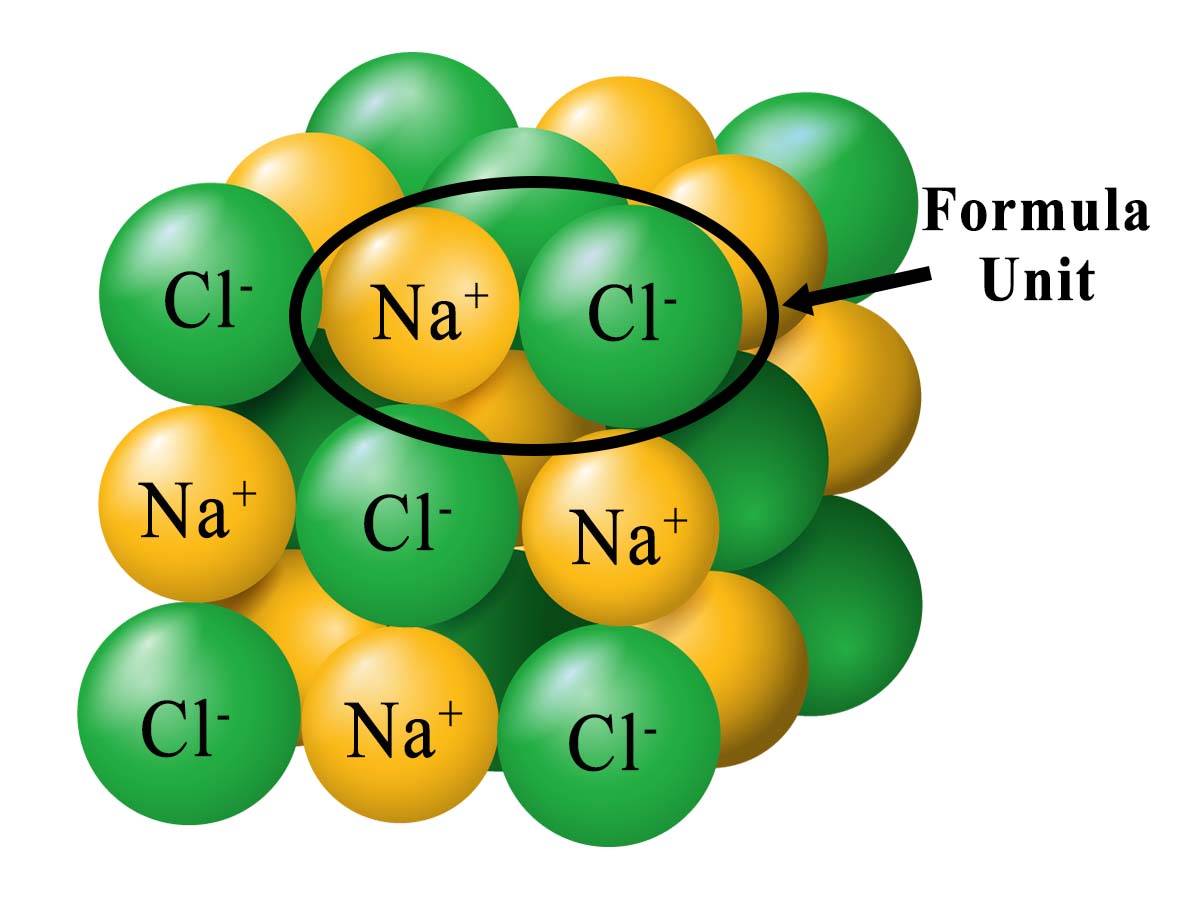 |
The number 6.02214076 × 1023 is called Avogadro's number. In this course, we will round it to four significant digits, as 6.022 × 1023. Notice that there is no unit associated with Avogadro's number. This is because it is simply the number of particles present, like referring to a quantity of 12 as a dozen.
The Mole and Avogadro's Number
The value of 1 mol of a substance is equal to Avogadro's number of its particles.
1 mol = 6.022 × 10 23 particles
Question
How is a mole similar to and different from a dozen?
The mole and the dozen are both counting units. A mole contains 6.022 × 1023 particles. A dozen contains 12 items.
Question
What is the relationship between a mole and Avogadro's number?
One mole of particles is equal to Avogadro's number, or 6.022 × 1023.
