In an earlier lesson, you learned that the atomic mass of an element is the weighted average mass of all the isotopes of that element. The atomic mass of an element is measured in unified atomic mass units (u) and is located underneath its chemical symbol on the periodic table.
The mass of an element measured in u is numerically equal to the mass of one mole of atoms of that element in grams. This mass in grams is called the molar mass. The molar mass of any substance is the mass in grams of one mole of the substance.
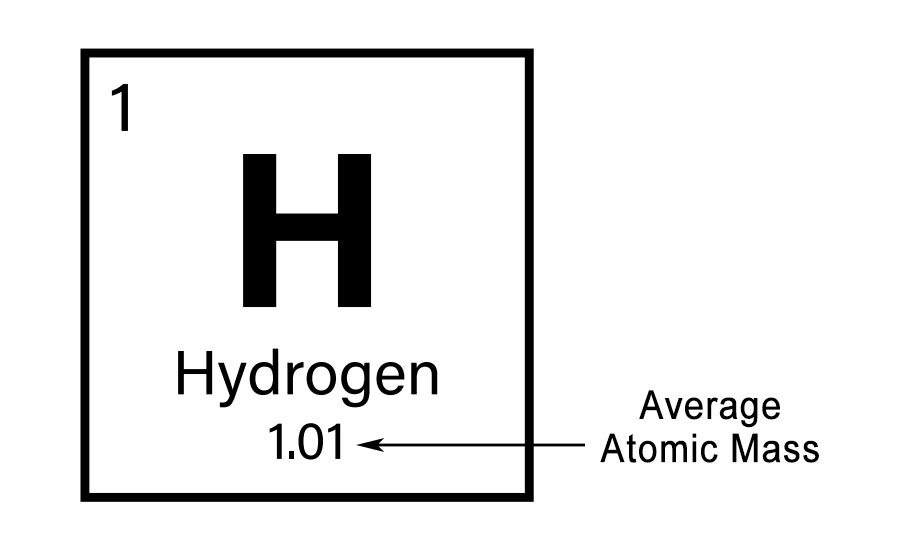
The molar mass of any element is numerically equal to its atomic mass and has the units \( \frac{\text{g}}{\text{mol}} \). Because the quantity for the molar mass of an element is the same as its atomic mass, it can be found on the periodic table. For example, the average atomic mass of hydrogen is 1.01 u. This means that one mole of hydrogen atoms will have a mass of 1.01 g. Thus, the molar mass of hydrogen is 1.01 \( \frac{\text{g}}{\text{mol}} \).
The molar mass of any compound is numerically equal to the sum of the atomic masses of all the elements in the compound. Determining the molar mass of a compound involves examining its chemical formula. Recall that the chemical formula for a compound tells you the number of atoms of each element in one unit of the compound. The number of moles of that element is the subscript for that element in the chemical formula. The subscripts also tell us how many moles of each element are in one mole of the compound.
Watch this video to learn how to determine the molar mass of compounds. Pay attention to the role that subscripts play in calculating the molar mass of compounds.
You may want to use the study guide to follow along. If so, click below to download the study guide.
We’ve learned that a mole is a specific quantity of something, usually atoms, ions, or molecules. We can use this very specific number, Avogadro’s number, to help us calculate the weights and quantities of substances involved in a chemical reaction.
Recall that Avogadro’s number, which is approximately 6.022 times ten to the twenty third, allows us to easily convert between unified atomic mass units and grams. For example, if an atom has a mass of 1.01 u, that would be hydrogen, then 1 mole of those atoms has a mass of 1.01 grams. See how easy that conversion was. So, the molar mass of hydrogen, that is to say, the mass of one mole of hydrogen, is 1.01 grams per mole.
Let’s look at some quick examples.
First, what is the molar mass of sodium chloride?
Well, the chemical formula for sodium chloride is NaCl. If you look at your periodic table, you’ll see that sodium has an atomic mass of 22.99 u, and chlorine has an atomic mass of 35.45 u. That means that one mole of sodium atoms has a mass of 22.99 grams – we call that its molar mass. And the molar mass of chlorine is 35.45 grams per mole. So, to find the molar mass of sodium chloride, we just have to add these values together. That gives us a molar mass of 58.44 grams per mole. So one mole of sodium chloride has a mass of 58.44 grams.
Now let’s do the same process, but with water. The chemical formula for water is H2O. The molar mass of hydrogen is 1.01 grams per mole, and the molar mass of oxygen is 16.00 grams per mole. But, remember that in a water molecule we have 2 hydrogen atoms, so we need to multiply this first number by 2. Now, if we add these values together, we see that the molar mass of water is 18.02 grams per mole.
Alright, one last example, but this is a tougher one. What is the molar mass of ammonium sulfate? Well, the chemical formula for ammonium sulfate is NH42SO4. That means we have 2 nitrogen atoms, each with a molar mass of 14.01 grams per mole. We have 4 times 2, or 8 hydrogen atoms, each with a molar mass of 1.01 grams per mole. There is just one sulfur atom, with a molar mass of 32.06 grams per mole, and there are 4 oxygen atoms, each having a molar mass of 16.00 grams per mole. If you multiply all these values out, and add them together, you’ll find that the molar mass of ammonium sulfate is 132.16 grams per mole.
Practice determining the molar masses of substances by completing this activity. Calculate the molar mass of each element or compound, then check your answer.
If you need a periodic table, click below to open an interactive periodic table or to download a PDF.


