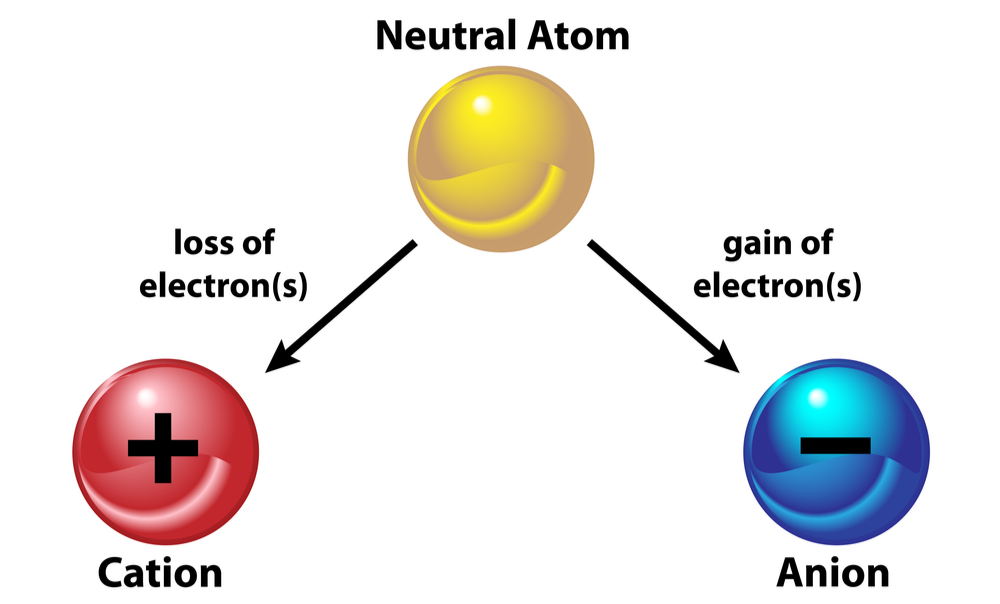
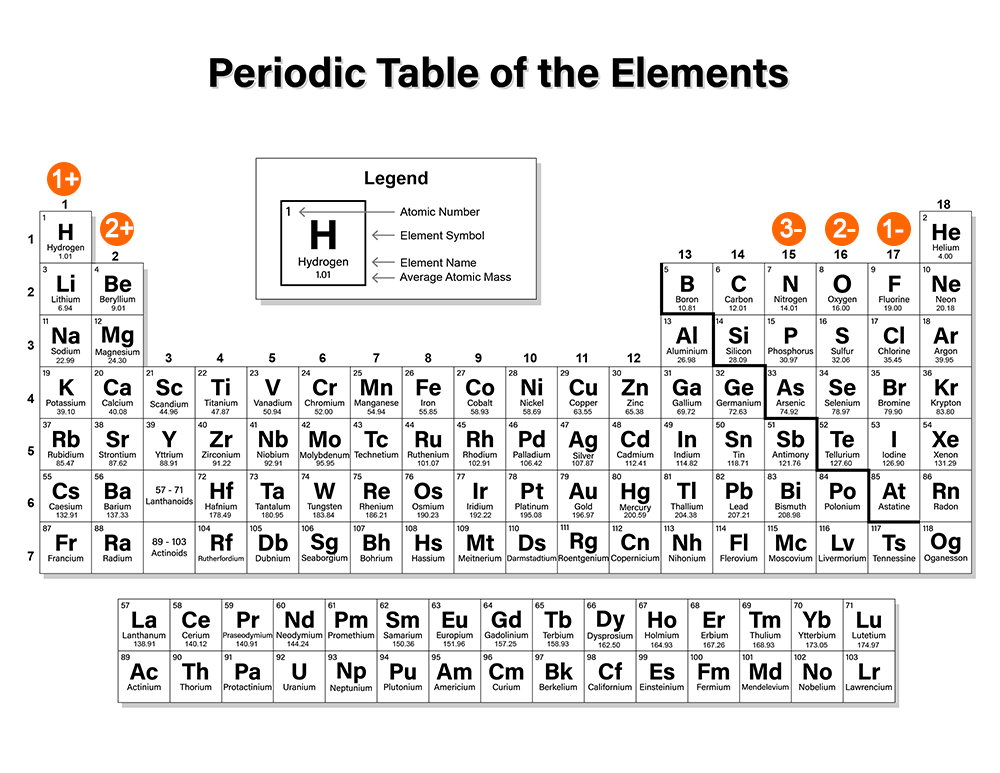
Earlier in this course, you learned about the process of ionization, in which atoms gain or lose electrons to form ions. The valence electrons are the electrons that are gained or lost in the formation of ions. View this slideshow to learn why atoms form positive and negative ions, and how ion formation relates to an element's position on the periodic table.
Complete this activity to practice determining whether elements will gain or lose electrons to form ions and identifying what charge those ions have. Choose the answer that correctly describes each ion.
If you need a periodic table, click below to open an interactive periodic table or to download a PDF.
Which statement describes how calcium (Ca) will form an ion?
- Calcium will gain two electrons to form a 2+ ion.
- Calcium will lose two electrons to form a 2+ ion.
- Calcium will gain two electrons to form a 2- ion.
- Calcium will lose two electrons to form a 2- ion.
Metals lose their valence electrons to form positive ions.
Metals lose their valence electrons to form positive ions.
Metals lose their valence electrons to form positive ions.
Metals lose their valence electrons to form positive ions.
Which statement describes how nitrogen (N) will form an ion?
- Nitrogen will lose five electrons to form a 5+ ion.
- Nitrogen will gain five electrons to form a 5- ion.
- Nitrogen will gain three electrons to form a 3- ion.
- Nitrogen will lose three electrons to form a 3+ ion.
Nonmetals gain enough electrons to fill their valence shell according to the octet rule. Gaining electrons makes a negatively charged ion.
Nonmetals gain enough electrons to fill their valence shell according to the octet rule. Gaining electrons makes a negatively charged ion.
Nonmetals gain enough electrons to fill their valence shell according to the octet rule. Gaining electrons makes a negatively charged ion.
Nonmetals gain enough electrons to fill their valence shell according to the octet rule. Gaining electrons makes a negatively charged ion.
Which statement describes how bromine (Br) will form an ion?
- Bromine will gain one electron to form a 1- ion.
- Bromine will lose one electron to form a 1+ ion.
- Bromine will gain seven electrons to form a 7- ion.
- Bromine will lose seven electrons to form a 7+ ion.
Nonmetals gain enough electrons to fill their valence shell according to the octet rule. Gaining electrons makes a negatively charged ion.
Nonmetals gain enough electrons to fill their valence shell according to the octet rule. Gaining electrons makes a negatively charged ion.
Nonmetals gain enough electrons to fill their valence shell according to the octet rule. Gaining electrons makes a negatively charged ion.
Nonmetals gain enough electrons to fill their valence shell according to the octet rule. Gaining electrons makes a negatively charged ion.
A mystery element formed an ion with a 1+ charge. What does this tell you about this element?
- The mystery element has seven valence electrons.
- The mystery element is in Group 17 on the periodic table.
- The mystery element gained one electron.
- The mystery element has one valence electron.
A positive one charge means the element lost one valence electron.
A positive one charge means the element lost one valence electron.
A positive one charge means the element lost one valence electron.
A positive one charge means the element lost one valence electron.
A new mystery element formed an ion with a 2- charge. What does this tell you about this element?
- The mystery element lost two electrons.
- The mystery element is in Group 16 on the periodic table.
- The mystery element is in Group 2 on the periodic table.
- The mystery element has two valence electrons.
A negative two charge means the element gained two electrons to fill its valence shell. A full valence shell has eight electrons in it, so the neutral element must have had six valence electrons.
A negative two charge means the element gained two electrons to fill its valence shell. A full valence shell has eight electrons in it, so the neutral element must have had six valence electrons.
A negative two charge means the element gained two electrons to fill its valence shell. A full valence shell has eight electrons in it, so the neutral element must have had six valence electrons.
A negative two charge means the element gained two electrons to fill its valence shell. A full valence shell has eight electrons in it, so the neutral element must have had six valence electrons.
Summary
Questions answered correctly:
Questions answered incorrectly: