When some molecules are more active and move faster than others, they are more likely to escape from the attraction of other molecules. Heat is the energizing force that causes some molecules to move around, speed up, escape, and so evaporate. (The illustration at the right represents a thermally agitated molecule.)
When molecules evaporate from a liquid, the average speed of the molecules in the rest of the liquid is lowered. This is because the faster molecules have evaporated, leaving the slower-moving molecules behind. Since heat is related to the movement of the molecules, the temperature of the liquid falls, as the molecules left behind are all moving more slowly, and heat is given off. This is why the evaporation of perspiration from your body on a hot day helps to keep you cooler.
Here is a way to think of it. Imagine you have a team of nine runners. Imagine the runners are all the molecules moving in a liquid. Some move faster, some move slower. Let’s say you then find the average speed of all the runners. Now imagine that the two fast runners are so fast that they decide to leave the team to form a different team.
 When some molecules are more active and move faster than others, they are more likely to escape from the attraction of other molecules. Heat is the energizing force that causes some molecules to move around, speed up, escape, and so evaporate. (The illustration at the right represents a thermally agitated molecule.)
When some molecules are more active and move faster than others, they are more likely to escape from the attraction of other molecules. Heat is the energizing force that causes some molecules to move around, speed up, escape, and so evaporate. (The illustration at the right represents a thermally agitated molecule.) 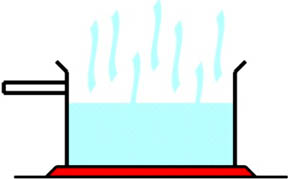 This is like two of the molecules moving so fast that they escape. You could then figure out the average speed of all the remaining runners, and it would be lower than the previous average. This is like the molecules left after the fast molecules left due to evaporation. The molecules that remain have a slower average rate of movement. Since they are slower, the temperature is lower.
This is like two of the molecules moving so fast that they escape. You could then figure out the average speed of all the remaining runners, and it would be lower than the previous average. This is like the molecules left after the fast molecules left due to evaporation. The molecules that remain have a slower average rate of movement. Since they are slower, the temperature is lower.
