 When objects are heated or cooled, they expand and contract. As you now know, when a solid, liquid, or gas is heated, its molecules move faster. They bump into each other as they need more room to move. Most solids spread out and get bigger as they are heated. This is called expansion. Different materials expand at different speeds and amounts. The amount of expansion depends on the composition of the material and on how much the temperature increases.
When objects are heated or cooled, they expand and contract. As you now know, when a solid, liquid, or gas is heated, its molecules move faster. They bump into each other as they need more room to move. Most solids spread out and get bigger as they are heated. This is called expansion. Different materials expand at different speeds and amounts. The amount of expansion depends on the composition of the material and on how much the temperature increases.Let’s picture molecules in a solid. The solid is a group of people standing close together, holding hands. Each person represents a molecule. The people start heating up, and trying to move around. They have a difficult time doing this, however, because they are packed tightly together. They jiggle, bump shoulders, and lean away from each other, inevitably expanding a little to take up more space. You can’t see it happening in a solid, but this is what is going on at the molecular level. Have you ever had difficulty opening a jar, and to make it easier you run the lid under hot water? This expands the lid (and not the jar because the lid is a better conductor) which loosens it.
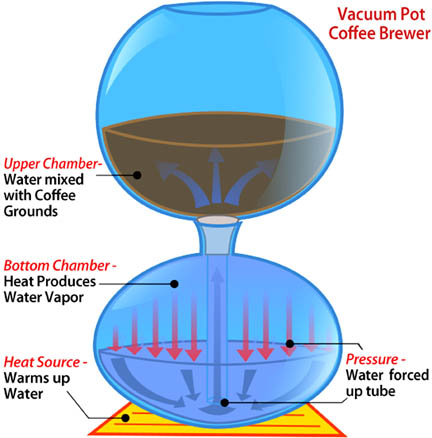
The iIllustration at the right is of a vacuum pot coffee brewer. This process makes the water go from the bottom to the upper chamber. The heat source warms up the water in the bottom chamber, produces vapor and builds up pressure. Vapor takes up more space than water because of the density of the molecules. To handle the pressure in the bottom chamber the water is forced up the tube (going north) to the upper chamber where it is mixed with coffee grounds.
Expansion
What does expansion depend upon?
The amount of expansion depends on the composition of the material and how much the temperature increases.
