Earlier in this course, you learned that all matter can be classified as either a pure substance or a mixture, and mixtures are classified as either homogeneous or heterogeneous. A homogeneous mixture is also called a solution.
A solution consists of at least one substance called a solute, evenly dispersed in another substance called the solvent. The solute is the substance in a solution that is present in the smaller amount. The solvent is the substance that is present in the larger amount.
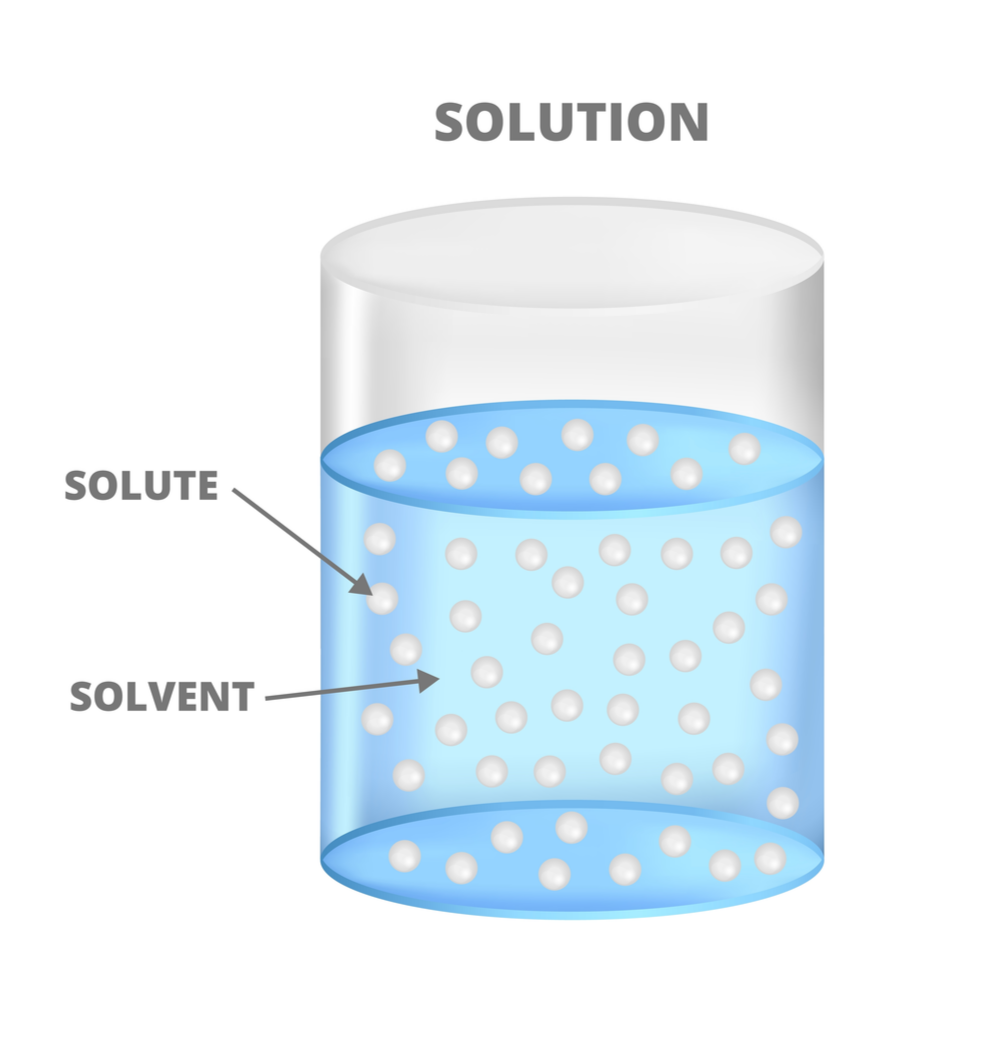
When some compounds dissolve, they dissociate, or separate into ions. For example, an ionic compound like sodium chloride (NaCl) will dissociate in water by separating into Na+ and Cl− ions. If the compound produces H+ ions during dissociation, we refer to that solution as an acid.
There are many acids that you may have encountered in your daily life, including these three examples:

Sulfuric acid is commonly used in car batteries.

The “fizziness” of sodas comes from dissolved carbon dioxide, which forms carbonic acid.

Dilute hydrochloric acid is used to maintain the pH of swimming pools.
Question
What is an acid?
An acid is a substance that produces H+ ions when dissolved in water.