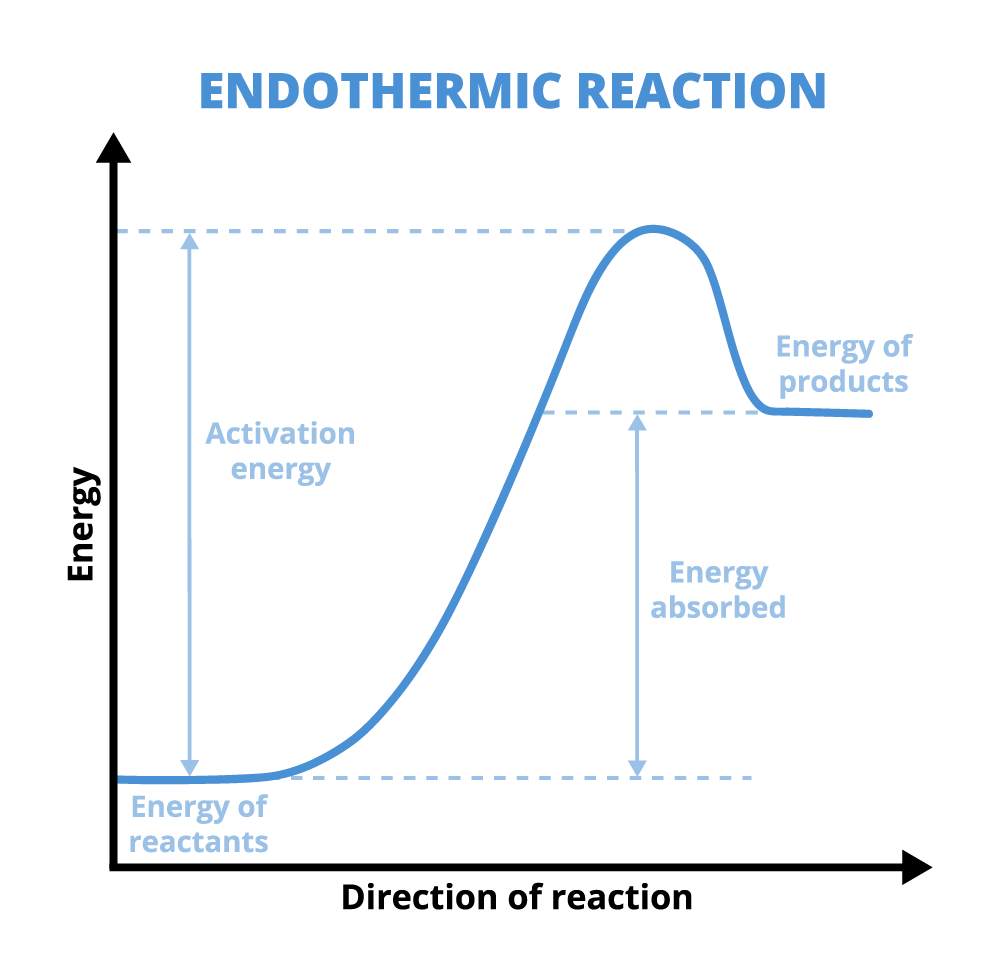
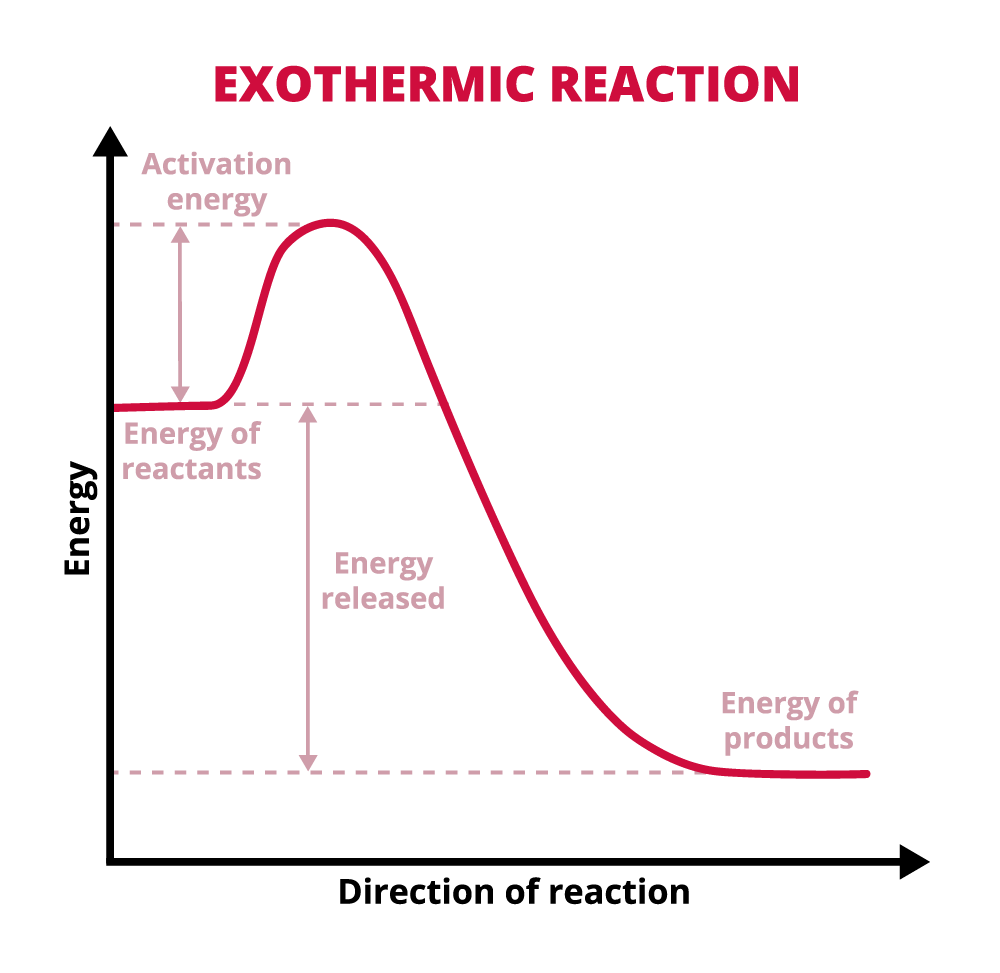
After the bonds are broken, new bonds will form. This is where there is a difference between endothermic and exothermic reactions. Explore the following tabs to learn the differences in energy for the two types of reactions.
If the bond energy of the products that are formed is higher than the bond energy of the reactants, then the reaction is an endothermic reaction. In this case, activation energy was put in to break the bonds in the products and the energy released when the products are formed is less than that activation energy.
What does an endothermic reaction do to the environment around the reaction?
The environment around the reaction cools down because energy was absorbed by the reaction. The energy absorbed is in the form of heat, thus cooling down the area around the reaction.
If the bond energy of the products that are formed is lower than the bond energy of the reactants, then the reaction is an exothermic reaction. In this case activation energy was put in to break the bonds in the products and the energy released when the products are formed is more than that activation energy.
What does an exothermic reaction do to the environment around the reaction?
The environment around the reaction heats up because energy was given off by the reaction. The energy given off is in the form of heat, thus heating up the area around the reaction.
Check your understanding of bond energy and type of reaction by answering the question on each slide. Then check your answer.
Which has more bond energy in the reaction happening in the MRE: the reactants or the products? Explain how you determined this.