Recall that potential energy is stored energy. One type of stored energy, called chemical potential energy, is energy that is stored within the chemical bonds of a substance. Bond energy is a measure of the bond strength of a chemical bond and is the amount of energy needed to break apart the atoms involved in the bond. A higher bond energy means that it is a stronger bond.
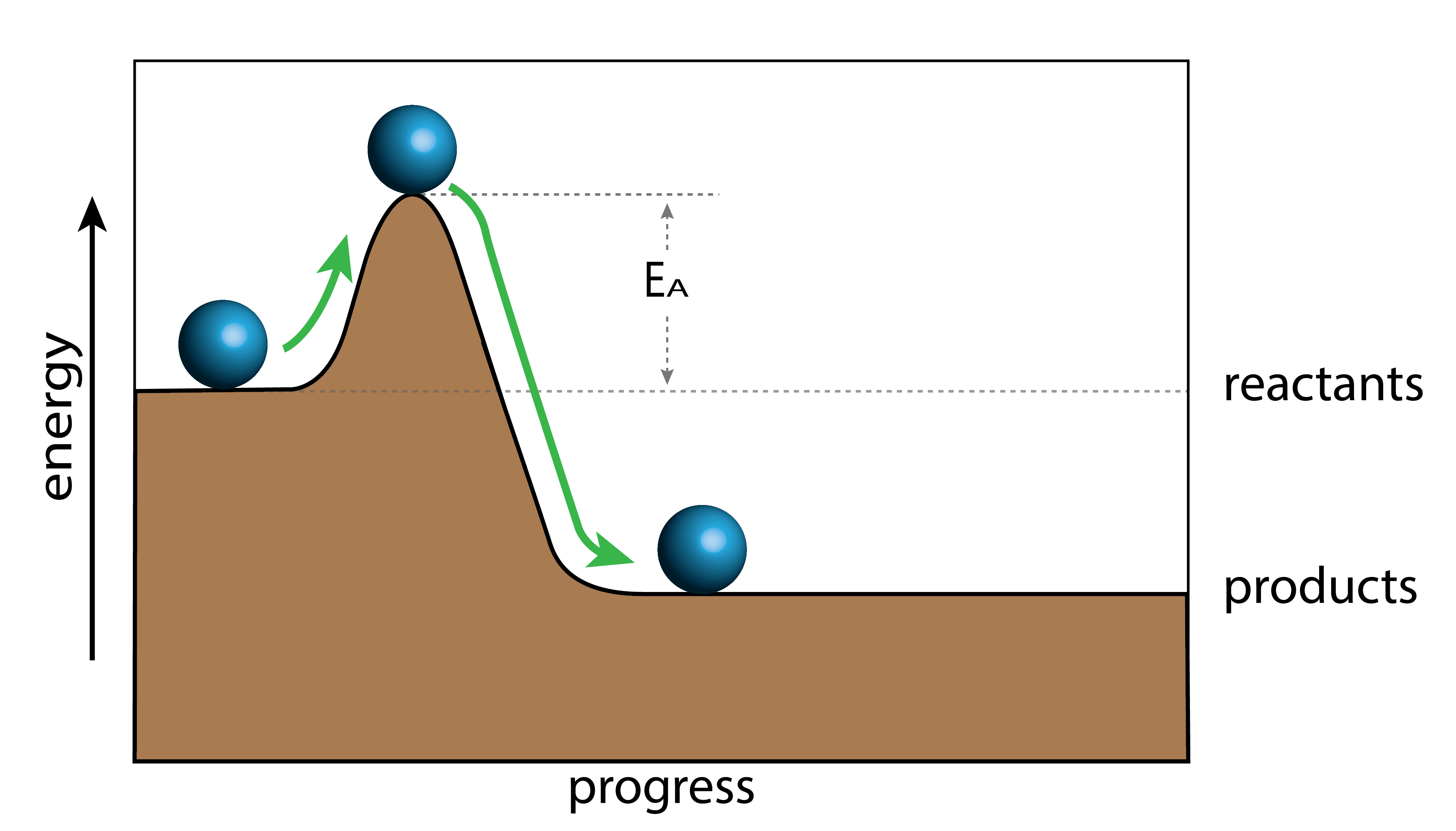
During a chemical reaction, the bonds in the reactants are broken and new bonds are formed to make the products. This process requires the addition and release of energy. It takes energy to break the bonds in the reactants. This is necessary for all reactions. The energy required to break these bonds is called the activation energy. You can think of the activation energy as a hill that the reactant particles must climb in order to form products.
Question
The energy difference between the ball at its highest point and the starting position of the ball represents the activation energy. This is represented by EA on the graph. This is the energy that is needed to break the bonds to start a reaction.