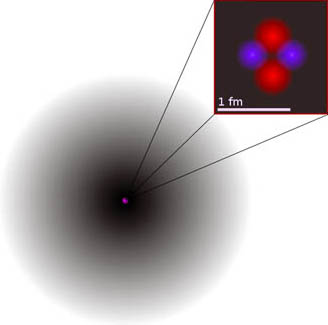
An atom is a small particle that is the basic building block of matter. Atoms are made up of even smaller particles: protons, neutrons,and electrons. The model of the atom has changed through the centuries as scientists gained new information.
The current model of the atom is the electron cloud model. In this model, the protons and neutrons make up a dense central core called the nucleus. A proton is a positively-charged particle in the nucleus and a neutron is a neutral or zero net charge particle in the nucleus. The nucleus of the atom is surrounded by a cloud of negatively-charged particles called electrons. The electrons are located some distance from the nucleus of the atom. As a result, most of an atom is empty space. Also, there is an equal number of protons and electrons in an atom. As a result, an atom does not have an electric charge.
Atom
What is an atom and what makes up an atom?
