Phase Change
What are phase changes?
Goal:
Goal:
Matter doesn't always stay in the same state. If the state of matter is the current form of something, such as a solid, liquid, or gas, then the phase change is the transition or movement from one state to another, such as from a solid to a liquid.
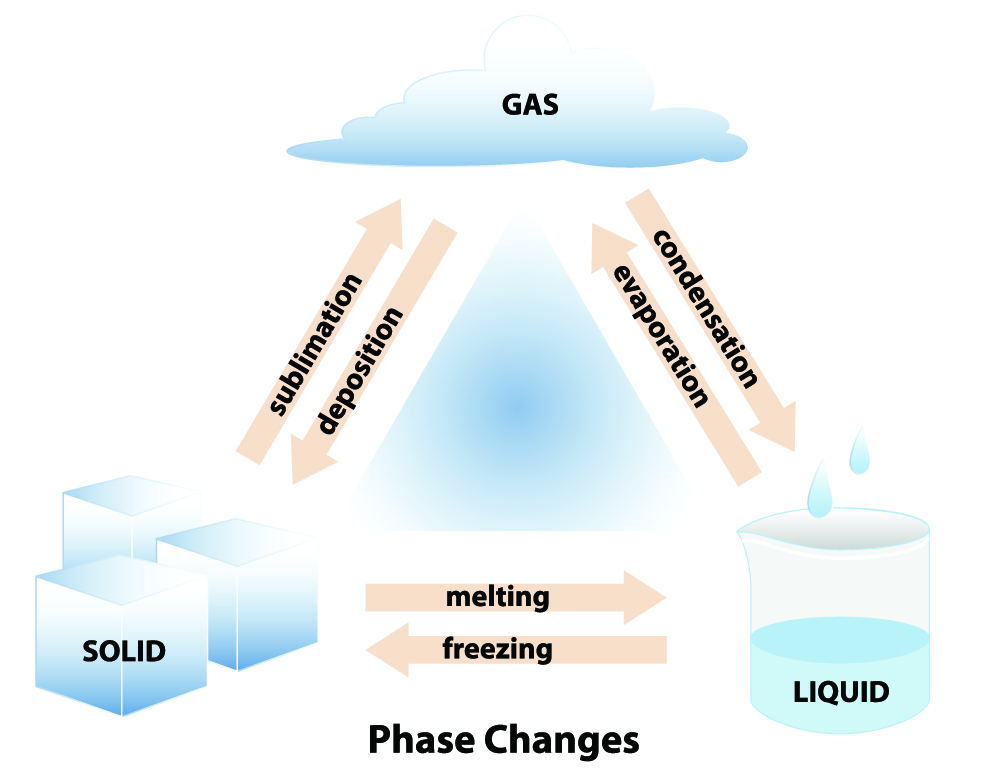
There are six phase changes:
- Freezing: liquid to a solid
- Melting: solid to a liquid
- Vaporization (Evaporation): liquid to a gas
- Condensation: gas to a liquid
- Sublimation: solid to a gas (no liquid phase)
- Deposition: gas to a solid (no liquid phase)
For a phase change to occur, heat energy needs to be absorbed or released. For example, when water atoms are frozen solid, they are packed in close to one another and are unable to move. Once the temperature of a solid begins to rise from a source of heat, the atoms in the solid gain energy in the form of heat energy. The heat energy gives the atoms energy, and they begin to move around more freely and bump into one another until they change into a liquid. Another phase change you may be familiar with is when you boil water. Heat is needed to give atoms energy in the form of heat energy, and in turn, matter will change phases. The opposite is true if you want to change something from a gas to a liquid, or a liquid to a solid. In that case, heat energy would have to be taken away.
Test your knowledge about phase changes by answering the questions below. Click each question to check your answers.